The global dialog about accessing fresh and clear water has to consider the impact of sustainable hydropower projects. This discussion is led by the International Hydropower Association (IHA). It is…
KMmaster® – Life sciences and pharmaceutical industry
Whether it be in industries such as the automotive industry, the chemical industry or the service sector, in quality assurance as an instrument of quality management, templates, activities and processes ensure that a product or service meets the defined specifications. A defined quality level should be achieved with the measures implemented.
Advantages and functions
|
||
Your benefits
|
Practical application examples
To support teams in the areas of help desk, call center and customer service, the KMmaster is used for:
- gathering and disseminating lessons learned, best practices etc.
- continuous improvement of products and processes
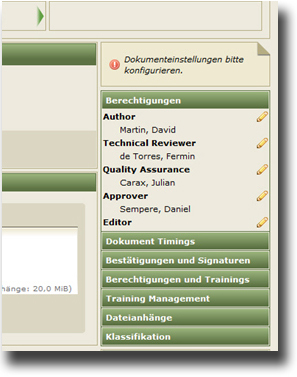
- control of documents through workflows
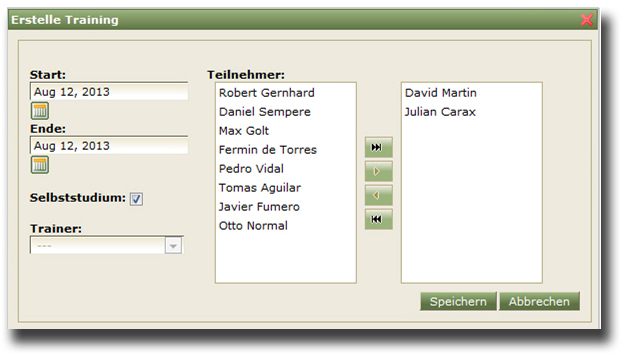
- cooperation in teams
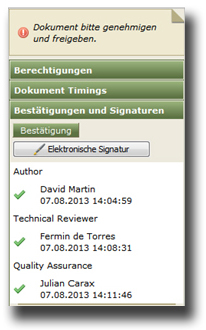
- monitoring and proof of quality
Find out more and familiarize yourself with our practical example from the field of life sciences.